San Francisco, CA
August 15-16, 1996
August 15, 1996
Welcome and Review of Agenda
Dale Schultz (DuPont), co-chair of the RTDF Permeable Barriers Action Team, welcomed participants to the meeting. Earlier in the day, the Action Team members visited two sites, Interstil, Sunnyvale, CA, and Moffett Federal Airfield, CA, where permeable barrier systems have been installed. Dale thanked Scott Warner (Geomatrix) and Chuck Reeter (Navy) for coordinating the visits to the respective sites. Both Scott and Chuck reported that "their" respective field demonstrations are successful to date. Dale indicated that the remainder of today's meeting will be primarily dedicated to discussions of permeable barrier field demonstrations. Degradation chemistry and engineering aspects of permeable barriers will be discussed during tomorrow's session.
Overview of California Regional Water Quality Control Board
Brad Job (California Regional Water Quality Control Board) provided an
overview of the California Regional Water Quality Control Board (CRWQCB) and
its role as it pertains to the implementation of innovative technologies. He
also discussed the justification for use of a permeable barrier at Interstil.
Brad indicated that a number of factors were conducive to the implementation of
an innovative technology at Interstil, including:
However, Brad noted that there were a number of difficulties to overcome
before the permeable barrier system was agreed upon by all concerned parties. A
number of neighboring property owners expressed concern about the viability of
a permeable barrier system and all PRPs were not initially agreeable to a
permeable barrier system. The concern of neighboring property owners was
significant because of possible offsite migration of contaminants if the
permeable barrier failed. Brad identified the following advantages to a
permeable barrier system:
Interstil appeared to be well suited for a permeable barrier and the system
appears to be functioning quite well. It has been operational since January
1995, and monitoring result show that water quality objective are being met.
Brad provided an overview of a number of Regional Water Quality Control Board
(RWQCB) statutes and regulatory policies that may impact the implementation of
a permeable barrier system, including:
Brad indicated that these statues and policies provide a mechanism to
approve an innovative technology. However, he suggested that some guidelines
may be prohibitive and noted that the CRWQCB aims to develop appropriate
cleanup goals by considering watershed management, receptor identification,
risk analysis, economic analysis, and data management. Growth issues will be
investigated- risk analysis and risk economics were identified as two such
issues. Brad identified the following regulatory initiatives to encourage the
development of innovative technologies:
Brad emphasized the importance of the Brownfields initiative and indicated that all of the above mentioned initiatives are, in part, intended to encourage PRPs to work together to solve their mutual problems. Brad added that efforts have been initiated to develop interstate/intrastate cooperation. Standards for multimedia risk evaluation, risk communication, and data sharing are under consideration and Brad noted that the interplay of thesethree factors is important to understand the hazards of a site.
In response to a participant's question, Brad indicated that zero-valent iron technology used successfully in numerous permeable barrier systems has not been certified. Chuck Reeter and Scott Warner agreed to determine if certification is advantageous to the permeable barrier technology. He noted that the demonstration design for Interstil was approved in approximately 3 months once the formal request was made. However, the CRWQCB and other regulatory agencies were involved in the developmental process, which expedited the approval process.
Overview of the ITRC
Paul Hadley (Cal-EPA) provided an overview of the ITRC and noted that Brad
Job accurately related a number of the ITRC objectives. The ITRC is a
state-lead organization promoting the development, demonstration, verification,
acceptance, and interstate deployment of innovative environmental technologies.
Stakeholder, tribal, and industry representatives participate in the ITRC,
which was formed in February 1995. Paul clarified that the industrial
participants are not ITRC members per se, but they are involved in most facets
of the ITRC. There is participation from the 27 states listed below, and Paul
indicated that additional states are encouraged to join:
| Arizona | Louisiana | Ohio |
| California* | Maryland | Oregon |
| Colorado | Massachusetts* | Pennsylvania* |
| Delaware | Minnesota | South Dakota |
| Florida | Nebraska | Tennessee |
| Illinois* | Nevada | Texas |
| Idaho | New Jersey* | Utah |
| Kansas | New Mexico | Washington |
| Kentucky | New York* | Wisconsin |
Paul indicated that six states (indicated by an asterisk above) have entered into a Memorandum of Understanding (MOU) to accept field data generated within one of the other states to promote technology transfer. The ITRC works actively with a number of federal agencies, including the Department of Energy (DOE), Department of Defense (DOD), and EPA. Several task groups have been formed since the inception of the ITRC, with the following focus areas- low temperature thermal desorption, in-situ bioremediation, plasma technology, site characterization and analysis penetrometer system (SCAPS). Paul noted that a "circuit rider," Rich Tomlinson, has also been identified for the ITRC. The ITRC In-situ Bioremediation Work Group has been working with the RTDF Bioremediation Consortium, and Paul, who is a member of the ITRC In-situ Bioremediation Work Group, believes that the relationship is mutually beneficial. A permeable barriers focus group will be formed in the near future, and Paul suggested that the RTDF Permeable Barriers Action Team work cooperatively with the ITRC Permeable Barrier Work Group. An objective of the permeable barrier focus group will be to obtain state regulatory and stakeholder acceptance of the technology. The Permeable Barriers Action Team will develop design protocols and it was suggested that the ITRC could provide a forum for review of the materials. It was suggested that the ITRC could define performance and criteria necessary to meet performance for SERDP Project #107 at Dover AFB, DE; Moffett Federal Airfield, CA; and Somersworth, NH. Steve McCutcheon (EPA/NERL), co-chair of the Action Team, indicated that he has spoken with Matt Turner (New Jersey Department of Environmental Protection), the anticipated chair of the ITRC Permeable Barriers Work Group, to identify a means for the two groups to work together and to discuss these suggestions and others. Steve also noted that Steve Shoemaker (DuPont) will interact with the ITRC on behalf of the Action Team. Jeff Marquesee (DOD) agreed that the RTDF and ITRC should interact regularly. In addition to the permeable barrier focus group, ITRC additional groups will be formed to: facilitate the integration of state efforts across working groups, review innovative state programs, and to increase electronic transfer of information.
Paul identified the following outputs of the existing focus groups:
Paul added that seven states, New Jersey, New Mexico, California, Nebraska, Utah, Louisiana, and Idaho, co-signed the SCAPS report.
Paul identified the following benefits of the ITRC to the participating
organizations:
A number of advantages were also identified for industry participants:
The ITRC endeavors to: become involved with real projects as they are designed, provide necessary regulatory input as early as possible, and seek as much regulatory consensus as possible to promote the "acceptance" of a technology. Paul noted that reaching an agreement on the definition of acceptance has been difficult. The ITRC seeks to participate in demonstrations with state regulatory and stakeholder involvement, develop protocols (define technology requirements and identify regulatory implications and requirements), and gather cost and performance data. "Performance" is another term that is difficult to define.
Update on Studies at Somersworth Sanitary Landfill
John Vogan (ETI) provided a brief update on column studies underway at the Somersworth Sanitary Landfill (Somersworth), Somersworth, NH. Elevated levels of volatile organic compounds (VOCs) are found in the groundwater. John indicated that one objective of the column studies is to examine the VOC degradation rate in order to arrive at an optimal field design. The column studies were performed using 100% iron as well as 50% iron mixtures. Different types of iron were explored, and John indicated that results obtained to date were, in general, consistent with previous experiences. However, John did note that Connelly-GPM iron exhibited similar results to that of Peerless iron, which was unexpected based upon the surface area of the materials.
A funnel and gate system (F&G) will be emplaced at Somersworth, and construction of the pilot-scale system is expected to begin fall 1996. A groundwater model is under development and near completion, which will be used to determine the necessary residence time. John indicated that various emplacement methods are under consideration.
Update on Studies at United States Coast Guard Support Center
Bob Puls (EPA/NRMRL) provided an update of ongoing studies at the United States Coast Guard Support Center (USCG) in Elizabeth City, NC. Bob acknowledged the participation of the University of Waterloo, the U.S. Coast Guard, and the State of North Carolina in the efforts at USCG. The USCG is located on the southern bank of the Pasquotank River. The demonstration site, outside of Hanger 79 at USCG, is approximately 60 feet from the river and was used for more than 30 years as a chrome plating shop. Bob indicated that acidic chromium wastes and associated solvents were discharged through a hole in the concrete floor and Bob indicated that the chromate plume has reached a receptor. A pilot test was begun in 1994 and has been monitored over the past 2 years. The primary objective of the study was to remove chromate contamination from the groundwater. Bob noted that two sources of iron were used in the pilot study- Masterbuilders and an iron developed at EPA's Kerr Laboratory. Hollow stem augers were used to emplace the iron in approximately 10-inch (diameter) columns. Bob indicated that a number of groundwater parameters were monitored. Dissolved oxygen (DO) decreased to non-detect levels; Eh lowered, pH was stable until March 1995, at which point it increased. Bob indicated that trichloroethene (TCE) levels were seen to decline and then rise. However, Bob noted that the field test was not designed to remediate chlorinated solvents so the residence time may not be appropriate for TCE remediation. In response, to a participant's question, Bob indicated that there is slight seasonal variation in the groundwater flow direction, which Bob speculated may account for a slight rise in chromate levels during one sampling interval. Elevated ferrous levels are seen within 1 meter of the columns.
Based upon the success of the pilot study, a full-scale effort was emplaced in June 1996. Two major plumes have been targeted in the full-scale effort- a chromate plume and a smaller chlorinated solvents plume. The objective is to reach regulatory limits for Cr(VI), TCE, cis-DCE, and vinyl chloride (VC). The emplaced iron wall was 50 m x 8 m x 0.6 m, used approximately 500 tons of Peerless iron, and cost about $300-350K to install. A trencher was used to emplace the wall, and Bob noted that the excavated materials were tested to determine if they were hazardous. Hazardous materials were relocated for treatment; non-hazardous materials were moved to another region of the USCG base. In response to a participant's question, Bob indicated the wall thickness (2 ft) is thicker than necessary. A 6-inch wall is necessary to address the chromate, and a 1.5 ft wall was needed to address the DCE. Bob indicated that initial plans called for installation of 2 parallel walls, both 1.5 ft thick, but that the design plan was modified to a single 2 ft wall. Operation and maintenance (O&M) costs are expected to be minimal, and the majority of the installation costs is associated with the iron. In response to a participant's question, Bob noted that the installation cost does not include site characterization or performance monitoring.
Performance monitoring devices have been employed with increased emphasis on accurate and efficient monitoring throughout the durationof the project. Bob indicated that tracer tests, microbial assessments, geophysics (to monitor wall integrity) tests will be performed. Four upgradient wells and six downgradient wells will be emplaced in addition to wells emplaced within the wall. Bob indicated that half of these compliance monitoring wells have been emplaced and that preliminary results indicate successful remediation of the chromate. Bob noted that conclusive data have not been collected to determine the efficacy of the system to remediate chlorinated solvents.
Update on SERDP Project #107
Jeff Stinson (Air Force) provided an update on the Strategic Environmental Research and Development (SERDP) Project #107, to be performed at Dover AFB, DE. The SERDP-project will investigate the degradation of chlorinated solvents using several different reactive media in a funnel and gate system (F&G). The RTDF Permeable Barriers Chlorinated Solvents Design Team met yesterday, August 14, 1996, to discuss the design and implementation of the permeable barrier system. Jeff indicated that four reactive media will be investigated via column studies in the near future, and that the most promising two or three of these media will be selected for use in the field demonstration. In addition, a long-term column study is under consideration, possibly at Dover AFB, to investigate the most promising reactive medium. A strawman design for the demonstration will be developed by early fall 1996. A draft protocol on permeable barriers is expected to the completed by January 1, 1997, and field construction should begin in March 1997.
The Design Team tentatively agreed to a system with a 10 ft gate for each reactive media Jeff noted that innovative emplacement technologies are under consideration to emplace portions of the F&G system. DuPont is investigating jet-grouting and Rich Landis (DuPont) indicated that the studies, to be performed at Dover AFB, should be completed by late fall 1996. Jeff indicated that a short list of potential reactive media has been identified, including: zero-valent iron with an oxygen scavenger such as troilite (to minimize precipitation/plugging), zero-valent iron with a buffering agent, such as pyrite, and Cercona pellets. Jeff added that the Remedial Cost Engineering and Requirements System (RACER) version 3.2 has been released and that it contains a permeable barriers module.
Overview of the Navy's Cost Modeling Module
Brad Schwartzman (Naval Facilities Engineering Southern Command) provided an overview of a permeable barriers costing model, dubbed SUCCESS, developed by the Navy. He indicated that the model is available to all DOD personnel, but it is not currently commercially available. Version 2.0 should be available by spring 1997, and Brad indicated that this version will be much more flexible than the current version. Although DOD will continue to use RACER, SUCCESS is expected to be used to a greater extent. The flow chart on the following page depicts the decision tree that the user follows to generate the cost estimate for a hypothetical permeable barrier system. Brad noted that the current module restricts emplacement to less than 50 ft because current emplacement costs are quite high for deeper systems. Brad demonstrated SUCCESS, which provided participants with a real-time example of its use.
Overview of Oak Ridge National Laboratory, Studies
Liyuan Liang (ORNL) provided an overview of the recent studies on radionuclides conducted at ORNL and DOE's Portsmouth Gaseous Diffusion Plant (PGDP). Liyuan noted that it is often difficult to treat radionuclides in-situ because they are not degraded, they must be removed from the subsurface. She has also been investigating chemical and physical methods for removal of VOCs. An interim report of the studies conducted at the PGDP Groundwater Remediation Facility is available. The preliminary design for these studies was completed in winter 1995; laboratory studies were performed in spring 1996; analyses of laboratory studies were completed during summer 1996. Additional studies are expected to occur in fall/winter 1996. The objective of the studies was to evaluate permeable barrier technology. Liyuan indicated that above-ground canister systems were used to investigate Peerless iron, Masterbuilders iron, and palladized iron. Liyuan indicated that the degradation rate observed in the system using palladized iron was 10 times faster than that of the other media, but also mentioned that it costs substantially more than the other investigated media.
There was no pumping per se into the canisters- they relied on gravity flow.
The dissolved oxygen in the groundwater was very low. Liyuan indicated that
hydrogen gas, bacteria build-up on the filter column, and iron oxide
precipitation in treatment system were seen in a number of the canisters.
Liyuan provided the following conclusions:
ENTER GRAPHIC
Liyuan provided the following operational observations:
Liyuan indicated that acid-washing is being explored to regenerate the clumped iron. Although it is not conclusive, a correlation has been observed between the presence of bacteria and gas build up.
Rich Helferlich (Cercona) provided a brief overview of Cercona's efforts, performed in conjunction with ORNL, to identify candidate reactive media suitable for a permeable barriers demonstration at ORNL's Y-12 Plant. Rich indicate that the hydrogeology present at the Y-12 Plant is challenging. The soil is primarily clay with a system of conduits, which creates a number of discreet plumes (as opposed to a common wide plume). The pH of the area is variable, generally acidic ranging from 1-2 to 4-5. A system of tributaries, which typically have a pH of 4-5, flow into a main creek (Bear Creek) downgradient of the contaminant source. Multiple contaminants, including nitrates, uranium, and chlorinated solvents are present.
Rich indicated that batch studies were performed to act as a screening
process for candidate media Candidate media tested include: a number of zero
valent iron materials, a number of iron oxide zeolites with mixed absorbents,
and a number of other absorbents from previous studies which had shown promise.
Preliminary results indicate that:
Rich indicated that Cercona pellets, which the same composition as a number of the Cercona foams used in the batch studies, will be also be investigated. Rich explained that the physical characteristics of the pellets are more amenable to the Y-12 site. In addition to the investigation of the pellets, a number of other prospective media will be investigated as well.
Update on Activities at Lowry Air Force Base
Bob Edwards (Booz-Allen &: Hamilton) provided preliminary results of a F&G system installed at the Fly Ash Disposal site at Lowry AFB, Denver, CO. Sheet pilings, installed to a depth of 20 ft last winter, were used as the funnel and the gate, composed of 90 tons of Peerless iron, was 10 ft wide. Pea gravel was also emplaced immediately before and after the iron to homogenize groundwater flow into and out of the gate. Bob indicated that performance monitoring wells in the wall indicate that chlorinated reduction is occurring- all VOCs are lowered to detection limits after traveling through two feet of wall, as indicated in the table below. Bob noted that ethene and ethane have also been detected, which suggests that dechlorination is occurring.
| Wall Cross Section (ft) |
PCE (PPB) |
TCE (ppb) |
cis-DCE (ppb) |
trans-DCE (ppb) |
VC (ppb) |
|---|---|---|---|---|---|
| 0 |
13.6 |
848 |
221 |
15.6 |
24.2 |
| 1 |
0.28 |
1.05 |
10.2 |
0.23 |
0.72 |
| 2 |
0.25 |
0.18 |
0.77 |
0.12 |
0.47 |
| 3.5 |
0.25 |
0.10 |
0.20 |
0.10 |
0.47 |
Bob indicated that the total cost of the F&G system is approximately $525K. Current cost projections suggest that the system needs to remain in operation for several years before it becomes cost effective and the cost per pound of chlorinated solvent removed will approach $24K after 10 years. This projection assumes that the iron is not replaced and that concentrations, degradation rates, and analytical costs are constant over this time. Bob suggested that as the design criteria become further refined, the overall project cost as well as the cost per pound of chlorinated solvents removed will be significantly reduced.
August 16, 1996
Discussion of Degradation Rates
Paul Tratnyek (Oregon Graduate Institute) discussed dechlorination degradation rates, why they differ between studies, and the degree to which they differ. Paul acknowledged Tim Johnson (Oregon Graduate Institute) and Michelle Scherer (Oregon Graduate Institute) and indicated that much of the presented material was developed by Tim and Michelle. Paul indicated that the Oregon Graduate Institute has compiled a catalog of references (journal articles, dissertations, books, etc.) on permeable barrier technologies and Paul noted that it is continually updated. There are currently 128 references. Paul asked that participants submit any missing references to him (see list of participants for contact information). Paul added that the list is available on the World Wide Web (Web) at http://cgr.ese.ogi.edu/ironrefs/, and also suggested that participants browse http://cgr.ese.ogi.edu/iron/, which provides an overview of the technology as well as a number of hotlinks to other permeable barrier Web sites.
In order to determine if independent laboratories were reporting similar results for observed rate constants (kobs) of chlorinated solvents, kobs was extracted from the literature and compared. Results published through November 1995 were used. Paul indicated that significant effort was needed to ensure that the compared kobs were reported in a similar fashion. In some situations, kobs was not reported, but Paul indicated that it was calculated where possible based upon reported data. Paul indicated that the kobs data, when plotted, appears to have no discernable trends. He noted that kobs data interpretation was difficult because of additional data was limited. Kobswas normalized to surface area, which lessened the kobs scatter, but Paul indicated that a discernable pattern was not evident. The figure to the left depicts ksa (kobs normalized to surface area). A number of other factors, such as pH, type of iron, initial concentration, etc., were investigated. Paul suggested that acid-washed iron may be have a higher rate constant, but cautioned that additional data is necessary. Paul did note, however, that the ksa(TCE) values are within an order of magnitude, except for one outlier. Paul suggested that this range should provide a benchmark forreported ksa(TCE) values. Paul noted that agreement is not as strong within a number of other compounds, but Paul suggested that the ranges suggested could be used as a guideline. Ksa was calculated using a correlation analysis and Paul indicated that the calculated ksa was comparable to existing data.
ENTER GRAPHIC...
Pathways and Products of Chlorinated Ethene Reduction by Metallic Iron
Dave Burris (Air Force's Armstrong Laboratory) presented an overview of the
pathways and products of chlorinated ethenes reduced by metallic iron. He
mentioned that the results presented today were developed in conjunction with
associates at Johns Hopkins University, ARA, New Mexico Tech, Washington State
University, and other collaborators. Batch studies were employed to investigate
the reduction of chlorinated ethenes by cast-iron. The batch system was
composed of the following: 5 g cast-iron, 0.1 g pyrite, 100 ml water, and 60 ml
headspace. Dave explained that using headspace allowed for easier capture of
degradation products. He indicated that a significant amount of the TCE is
sorbed onto the iron, which is often unaccounted for in mass balance
considerations. He suggested that other phases than just the aqueous phase need
to be considered or a skewed pictorial may result. Transient conditions need to
be identified and considered. Dave indicated that approximately 40 pore volumes
are necessary to achieve steady-state. Dave stated that the primary types of
reactions involved in the reductive process are:
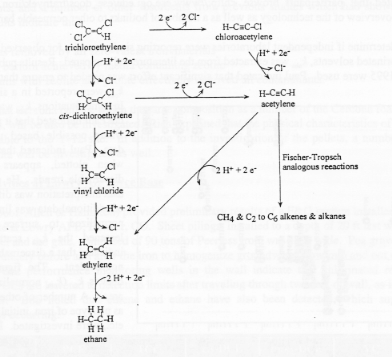
The Fischer-Tropsch reactions account for the production of alkanes and alkenes and suggested that they account for other products as well. Possible pathways for reduction of chlorinated solvents are depicted below.

Dave provided the following summary comments:
Discussion of Reactive Media Choices and Mechanisms
Tim Sivavec (GE) provided an overview of efforts by GE to investigate
reactive media and mechanisms. He indicated that GE's research goals are to:
The following table identifies work in-progress in the field of permeable barriers; Tim noted that it is not necessarily an inclusive listing.
| Contaminant | Treatment Media | Work Location (Field Work) |
|---|---|---|
| Chlorinated Solvents |
Zero-valent iron, low-cost, granular recycled iron, Cercona foam/pellets |
University of Waterloo (Yes); ETI (Yes); EPA/Athens; Oregon Graduate Institute; GE (Yes); DOE (Yes); Dupont (Yes); Monsanto; Air Force/Tyndall AFB |
| Metallic couples, metal- plated iron filings |
Sweeney patents, Fe/Pd: ORNL, Univeristy of Arizona (Yes); Fe/Cu: Monsanto, DuPont; Fe/Ni: University of Waterloo; Fe/Ni: GE |
|
| Iron sulfide minerals | Pyrite, FeS2: Reinhard, Stanford Universtiy; pyrite as a buffer: EPA/Athens; troilite, FeS, and other FexSy: GE |
|
| Other redox-active minerals | Magnetite, ilmentite, chalcopyrite: GE; chalcopyrite, CuFeS2: Michigan Tech Univ.; Fe(II) - midofied magnetite: EAWAG |
|
| Colloidal iron | Pacific Northwest Laboratory (Yes) | |
| Reduction of aquifer materials with dithionite |
Pacific Northwest Laboratory | |
| Granular irons plus sorbents | GAC: University of Waterloo; GAC and polymeric sorbents: GE |
|
| Sorption barriers | Surfactant-modified clays: Tyndall AFB; zeolites: New Mexico Tech |
|
| Cr(VI) | Zero-valent iron | University of Waterloo (Yes); EPA/Kerr Lab (Yes); ORNL |
| Dithionite | Pacific Northwest Laboratory (Yes) | |
| H2S | DOE, Sandia National Laboratory (Yes) | |
| Uranium/Mill Tailings |
FeCl3-FeOOH | DOE/Rust Geotech |
| Peat | DOE/GJPO/Rust Geotech | |
| H2S | Wyoming | |
| Surfactant-modified zeolites |
New Mexico Tech | |
| PAHs | Granular activated carbon | University of Tubingen (Yes) |
| Radionuclides | Zero-valent iron | ORNL/LMES |
| Zeolites | DOE/PNL/Hanford (Yes) | |
| Pesticides | Zero-valent iron | EPA/Athens |
| Chlorinated Aromatics |
Fe/Pd | ORNL |
| Low As/High Phosphate |
NaHCO3, Mg(OH)2, CaSO4 | DuPont |
| Nitroaromatics | Zero-valent iron; Fe(II)- treated magnetite |
Oregon Graduate Institute; EAWAG |
Tim provided the following criteria for selection of an iron metal:
The following key parameters affecting dechlorination rates were identified:
Tim indicated that mineral precipitation, which leads to loss in iron zone permeability, is a leading cause of performance degradation. Mineral precipitation is due to: (1) plugging at the system entrance, and (2) loss of porosity throughout the iron zone. GE column flow tracer analysis has suggested that losses of porosity are typically 10% to 25%. Tim indicted that there is a linear relationship between the iron metal surface area and the TCE reduction rates. He provided a comparison of TCE reduction rates in batch and column studies performed by GE, as depicted in the following table.
| Systen | Water | Type of Iron | M2/L | ks*(Lh-1s-2) |
|---|---|---|---|---|
| Batch | DI | Peerless, 50 mesh to dust |
180 |
1.9 x 10-4 |
| Batch | DI | Peerless, 20 mesh to dust |
126 |
1.7 x 10-4 |
| Batch | DI | Peerless, 8 plus 50 mesh |
- |
1.3 x 10-4 |
| Batch | Site gw #1 | Peerless, 8 plus 50 mesh |
- |
2.5 x 10-4 |
| Column | Site gw #1 | Peerless, 8 plus 50 mesh | 3810 |
1.2 x 10-4 |
| Column | Site gw #2 | Peerless, 8 plus 50 mesh | 4210 |
2.4 x 10-4 |
| Column | Site gw #2 | Peerless, 8 plus 50 mesh | 4760 |
5.7 x 10-4 |
* - represents surface-area normalized first order rate constant.
Tim also provided the following summary of GE's groundwater column treatability studies:
| Column Media | Pore Volumes Treated |
TCE Half- life (h) |
Effluent pH |
|---|---|---|---|
| 100% granular iron | 225 |
1.8 |
8.6 |
| Granular iron: FeS (92:8) | 400 |
2.0 |
7.8 |
| 100% granular FeS | 160 |
0.9 |
7.3 |
Tim suggested that controlling the pH may allow Fe(II) migration downgradient, which could extend the reactive zone downgradient as well. GE has also performed work with bimetals, and Tim provided a brief overview of work in this field. He noted that a number of different sources for minerals are being investigated, and that the cost-effectiveness of the reactive media is important. (i.e., palladized iron exhibited a half-life 45x less than iron, but costs approximately 200x more per ton).
Overview of Monsanto's Bimetallic Reductive Dechlorination Studies
Robert Orth (Monsanto) provided an overview of Monsanto's iron and bimetallic reductive dechlorination studies. He indicated that the objectives of the studies are to: (1) examine mechanisms of dechlorination with iron and iron couples (bimetals); (2) determine what changes could be made to improve the rate of the reaction; and (3) obtain mass balance for products. In general, Robert indicated that an increase in the rate of corrosion results in an increase of the rate of dechlorination; this can be achieved by control of the pH, and use of galvanic couples (mixed metals or metals plated on the surface of the iron).
Robert provided the following overview of the experimental and analytical
design:
Robert provided a brief overview of the corrosion process involved in the dechlorination of solvents by zero valent iron. He indicated that the rate determining step is dependent upon the catalytic properties of the iron surface. Any metal with higher reduction potential will accelerate the dehalogenation process. Robert indicated that bimetals increased the rate in the following order based upon Monsanto's laboratory studies: palladium > tin > silver > copper > gold > 99.2% iron > 99.999% iron. Robert provided the following data indicating the influence of surface coverage on rate:
| Calculated Monolayer of Copper of Iron Surface |
Observed Half-life (h) |
|---|---|
| 0 |
111 |
| <0.59 |
30 |
| <7 |
37 |
| <30 |
38 |
It appears that a combination of pathways are occurring. Robert indicated
that the mechanism changes as the bimetallic changes. Robert provided the
following summary statements:
Nickel-Iron as Reactive Material
John Vogan provided a brief overview of column studies performed by EnviroMetal Technologies, in collaboration with the University of Waterloo, to investigate the efficacy of nickel/iron to dechlorinate solvents. John indicated that nickel was observed only in the effluent of the first 10 pore volumes. ETI is investigating a number of nickel/iron alloys to identify the optimal material. John indicated that the majority of column study results are preliminary at this time, but are encouraging. In addition to recent column studies, an above-ground canister study, using nickel-plated iron, was initiated at a site in Wayne, NJ, during November 1994. John noted that it was difficult to obtain sufficient quantities of nickel-plated iron to perform the study. Approximately 2 tons of reactive media was used at $2-4K/ton. John noted that carbonate precipitate was a major concern and a number of measures were taken to minimize precipitation. In response to a participant's question regarding hydrogen gas build-up, John Vogan indicated that a sampling vent was used to release gas build-up within the NJ canister study.
Kinetics of TCE Reduction by Iron in the Presence of Soil
Alan Rabideau (SUNY Buffalo) provided an overview of efforts at SUNY
Buffalo, with the support of DuPont, to investigate the kinetics of TCE
reduction by iron in the presence of soil. Alan acknowledged the support of
DuPont. The motivation for the studies were: (1) diffusive transport of
dissolved contaminant in conventional soil/bentonite slurry walls is expected
to produce breakthrough within the system design life, (2) greatly enhanced VOC
degradation occurs in the presence of Fe0, and (3) a half-life of
less than 1 year would significantly reduce VOC transformation in
soil-bentonite walls. Alan indicated that batch and column studies were
performed. The following batch study results were provided:
Regarding the column study, Alan indicated that hydrogen production was
observed and that 3-7 weeks were required before steady-state conditions were
achieved. TCE breakthrough was not seen in the column studies; however, Alan
indicated that the majority of the column studies were only recently completed
and that complete analysis has not been performed. Follow-up batch studies,
however, have been completed. Alan reported the following results:
Alan identified a number of potential mechanisms to address the role of soil
in the degradation of TCE:
Overview of Emplacement Technologies for Permeable Reactive Zones
Rich Landis (DuPont) provided an overview of emplacement technologies for
permeable reactive zones.
Rich identified the following issues regarding emplacement techniques:
Rich identified the following path forward for DuPont's evaluation of
emplacement technologies:
Mineral Precipitation and Hydraulic Issues of Permeable Reactive Zones
Pat MacKenzie (GE) discussed mineral precipitation and hydraulic issues as they pertain to permeable reactive zones. Pat indicated that hydraulic losses are due to either plugging or porosity losses. Porosity losses are due to mineral precipitates, such as FeCO3 and CaCO3, and are typically found throughout the length of a column, canister, or in-situ treatment zone. Plugging is typified by an orange/brown solidified mass and is caused by the cementing of particles by ferric (oxy)hydroxides. Plugging usually causes rapid pressure rise, and is most commonly found near the inlet port of a column or canister. Plugging may divert groundwater flow around an in-situ treatment zone. Pat indicated that high DO levels contribute significantly to precipitation, and must be accounted for in either an in-situ or ex-situ study. GE is investigating the use of oxygen scavengers to reduce DO levels prior to contact with the reactive media The oxygen scavenger is typically emplaced as a layer immediately in front of the reactive media, and Pat indicated that GE is investigating use of removable cassettes, which may then be replaced if necessary.
Pat provided additional details regarding mineral precipitation in
in-situ treatment zones, that it is caused by:
Pat explained that the impact of the precipitation is as follows:
Pat indicated that GE performed column studies to measure porosity losses over time. She noted that a nonsorbing tracer was used to determine porosity losses and that a 10% loss in porosity was seen after 382 pore volumes, equivalent to 9.8 years in real-time. Pat indicated that a small porosity loss can have a significant impact on zone permeability. Because of this, Pat suggested that reactivity/porosity losses should be carefully monitored at the entrance of an in-situ treatment zone.
General Discussion
Dale Schultz initiated a general discussion of the earlier presentations and of issues relevant to the Permeable Barriers Action Team. Dale suggested that the Action Team identify needs to further permeable barriers as a remediation technology. Don Marcus (EMCON) felt that bimetals are promising, and asked if any long term column studies had been performed. John Vogan was unaware of any bimetal column studies where greater than 200 pore volumes were used, but Tim Sivavec mentioned that GE is performing a Ni/Fe column study and expects that 500 pore volumes will be used.
Tim Sivavec suggested that sulfur compounds seem to negatively impact the effectiveness of bimetals. John Vogan indicated that he has experienced biofouling, but did not observe indications of increased sulfate reducing microbial populations, which he did note was unusual. Pat MacKenzie indicated that GE has seen a significant amount of microbial activity in groundwater, but has not analyzed the effluent. John indicated that this phenomenon will be investigated more closely in the future. In response to a participant's question, John suggested that several months are necessary before a field system stabilizes to a steady-state. He noted that an in-flux of contaminated groundwater into the reactive barrier often occurs during construction, which adds to the time needed to achieve steady-state. Pat MacKenzie suggested that 2 months are sufficient to flush the field system. Dale Schultz suggested that it is dependent upon groundwater velocity and other key parameters.
Scott Warner asked if any Action Team members had performed column studies with a geometry more similar to field systems. Pat MacKenzie indicated that GE considered non-circular columns, but they were difficult to engineer. Stan Morrison (Rust Geotech) indicated that a "tank" system was developed by DOE and Rust Geotech to better mimic field conditions, but the system was difficult to operate. Pat MacKenzie noted that a reliable method to collect and transfer groundwater from the subsurface to the laboratory without altering its characteristics has not yet been identified. Similarly, a participant suggested that there are a number of unresolved issues regarding engineering scale-up from the laboratory to a field system. Scott Warner felt that engineering and hydraulic conditions are often considered a lower priority than the degradation chemistry. Rich Landis noted that there is often limited hydraulic data to engineer a field system. A participant suggested that performance monitoring is more critical if an innovative emplacement technique is used as the integrity of the wall may be in question. Dale Schultz agreed that performance monitoring is very important in thin walls, such as those created by high-pressure jetting or tremie tubes.
Concluding Remarks
Dale Schultz thanked presenters and participants and stated that a significant amount of information was shared during the meeting. Dale noted that dechlorination chemistry has been a leading topic of the day's presentations and a number of participants felt that follow-up presentations would be of interest at a future meeting. Additional participants suggested that a discussion of hydraulics/scale-up from the laboratory to the field would be of interest. The Action Team agreed to meet in Denver, CO, on December 11-12, 1996. Bob Stone (EPA\Region VIII) noted that the U.S. Geological Survey is leading a F&G field demonstration outside of Denver, CO, and suggested that the Action Team could visit the demonstration at the next meeting. Dale asked participants to provide any additional topics for the future meetings so that they may be considered.
The following action items were identified during the meeting:
Matt Turner:
Chuck Reeter, in coordination with Scott Warner:
Ed Marchand will provide 2 copies of slides and overheads of the Lowry AFB pilot installation and results (including economics).
John Vogan will provide 2 copies of slides and overheads of additional pilots with which ETI has been involved, and will also provide cost and design information.
Mark Searles will provide Steve McCutcheon with a monthly status report on each of the Action Items.
| Mr. William Baugman Dr. Robert Bowman Dr. David Burris Ms. Darcy Byrne Mr. Kirk Cantrell Ms. Sandy Clavell Mr. Bob Edwards Dr. Steven Fann Ms. Stephanie Fiorenza Dr. Arun Gavaskar Mr. Will Goldberg Dr. Neeraj Gupta Mr. Rich Helferich Mr. Grant Hocking Dr. Ron Holser Mr. Brad Job Dr. Rich Landis M. Liyuan Liang Mr. Gus Lo Dr. Patricia MacKenzie Mr. Steve Mangion Major Ed Marchand Mr. Donald Marcus Mr. Jeff Marqusee Dr. Steven McCutcheon Dr. Stan Morrison Mr. Mark Noll |
Ms. Mary North-Abbott Major Jeff Ogden Dr. Bob Olfenbuttel Dr. Robert Orth Second Lieutenant Dennis O'Sullivan Ms. Carey Peabody Dr. Robert Puls Mr. Kenneth Quinn Mr. Alan Rabideau Mr. Chuck Reeter Mr. Peter Russell Dr. Bruce Sass Mr. Michael Schnarr Dr. Dale Schultz Mr. Mark Searles Dr. Timothy Sivavec Mr. Chris Smith Mr. Bob Starr Mr. Richard Steimle Captain Jeff Stinson Mr. Robert Stone Mr. Brad A. Schwartzman Dr. Paul G. Tratnyek Mr. John Vogan Mr. Scott Warner Mr. Stephen White Mr. Randy Wolf Mr. Darrin Wray |
| Dr. Jim Anderson Mr. Frank Anderson Dr. Martin Bell Dr. David Blowes Ms. Beverly Campbell Mr. Cliff Casey Mr. Skip Chamberlain Mr. Dean Chartrand Dr. Chien Chen Mr. Mark Cipollone Mr. Richard Conway, P.E. Dr. Jim A. Davis Dr. Tom Early Dr. David Ellis Dr. John Fruchter Dr. Bob Gillham Dr. Neil Gray Dr. Baohua Gu Dr. Johnson Haas Mr. Joseph Hailer Dr. Kirk Hatfield Mr. Conrad Ingram Mr. Peter Jeffers Dr. Erica Jonlin Mr. Mark Kaminski Mr. Joe King Dr. Gary Klecka Mr. George Korfiatis Mr. John Koutsandreas Mr. Gus Lo |
Mr. Tom Malloy Dr. Dianne Marozas Ms. Lynn McCloskey Mr. Peter McMahon Ms. Alanna Mitchell Ms. Jennifer Nelson Ms. Stephanie O'Hannesin Dr. Ian T. Osgerby Mr. Philip Palmer, P.E. Mr. Anthony V. Palumbo Mr. Greg Penland Mr. Edward Pesce Mr. Gene Peters Mr. Mark Phifer Dr. Will Robertson Mr. Michael Royer Mr. H.G. Sanjay Mr. Bob Schenck Mr. Richard Scheper Mr. Steve Shikaze Mr. Stephen Shoemaker Dr. David Smyth Mr. Gregg Somermeyer, P.E. Dr. Daniel Stone Mr. Marland Thurston Mr. Chuck Turick Mr. Harry Van den Berg Dr. Joyce Whang Mr. Steve Winters Dr. Lee Wolfe |